Dietary supplements intended for men have been withdrawn from circulation by Nébih
The National Food Chain Safety Authority (Nébih) has withdrawn dietary supplements intended for men containing pharmaceutical ingredients from the market. The measure affects all batches of the Anaconda for men, Kinger and Titán Power Classic products, regardless of the shelf life – Nébih told MTI on Tuesday.
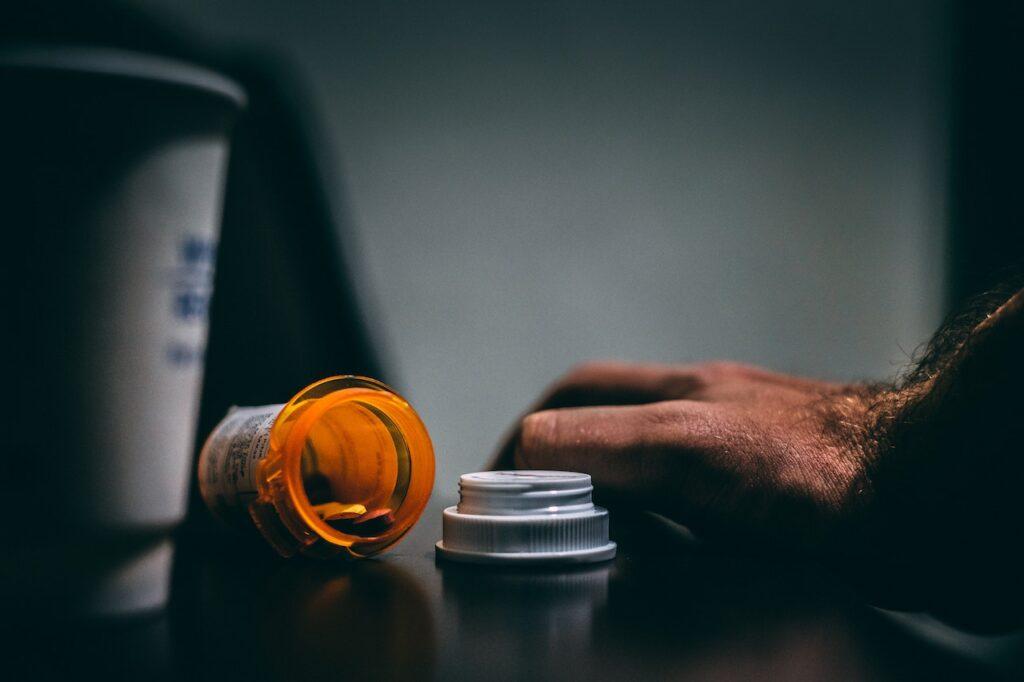
According to the announcement, three dietary supplements intended for men came to the attention of the authority during the annual monitoring sampling. Tests by the National Pharmaceutical and Food Health Institute of the affected products revealed active pharmaceutical ingredients.
The active pharmaceutical ingredients involved are compounds used in the therapy of erectile dysfunction (potency disorder) due to their inhibitory effect on the phosphodiesterase-5 enzyme (PDE-5). The release of preparations containing this active ingredient in Hungary is subject to a medical prescription, while dietary supplements – according to both EU and Hungarian regulations – are considered food and the use of medicinal substances in them is prohibited, they wrote.
Related news
Useful information on filling in the new data fields of the spraying log
🎧 Hallgasd a cikket: Lejátszás Szünet Folytatás Leállítás Nyelv: Auto…
Read more >Nébih: Bird flu confirmed again in Bács-Kiskun County
🎧 Hallgasd a cikket: Lejátszás Szünet Folytatás Leállítás Nyelv: Auto…
Read more >Bird flu confirmed again in Bács-Kiskun County
🎧 Hallgasd a cikket: Lejátszás Szünet Folytatás Leállítás Nyelv: Auto…
Read more >Related news
The 2026 Marketing Diamond Awards have been presented
🎧 Hallgasd a cikket: Lejátszás Szünet Folytatás Leállítás Nyelv: Auto…
Read more >FAO food price index rises again in February after five months
🎧 Hallgasd a cikket: Lejátszás Szünet Folytatás Leállítás Nyelv: Auto…
Read more >MBH quick analysis: The snow situation shaped store traffic in January
🎧 Hallgasd a cikket: Lejátszás Szünet Folytatás Leállítás Nyelv: Auto…
Read more >







